
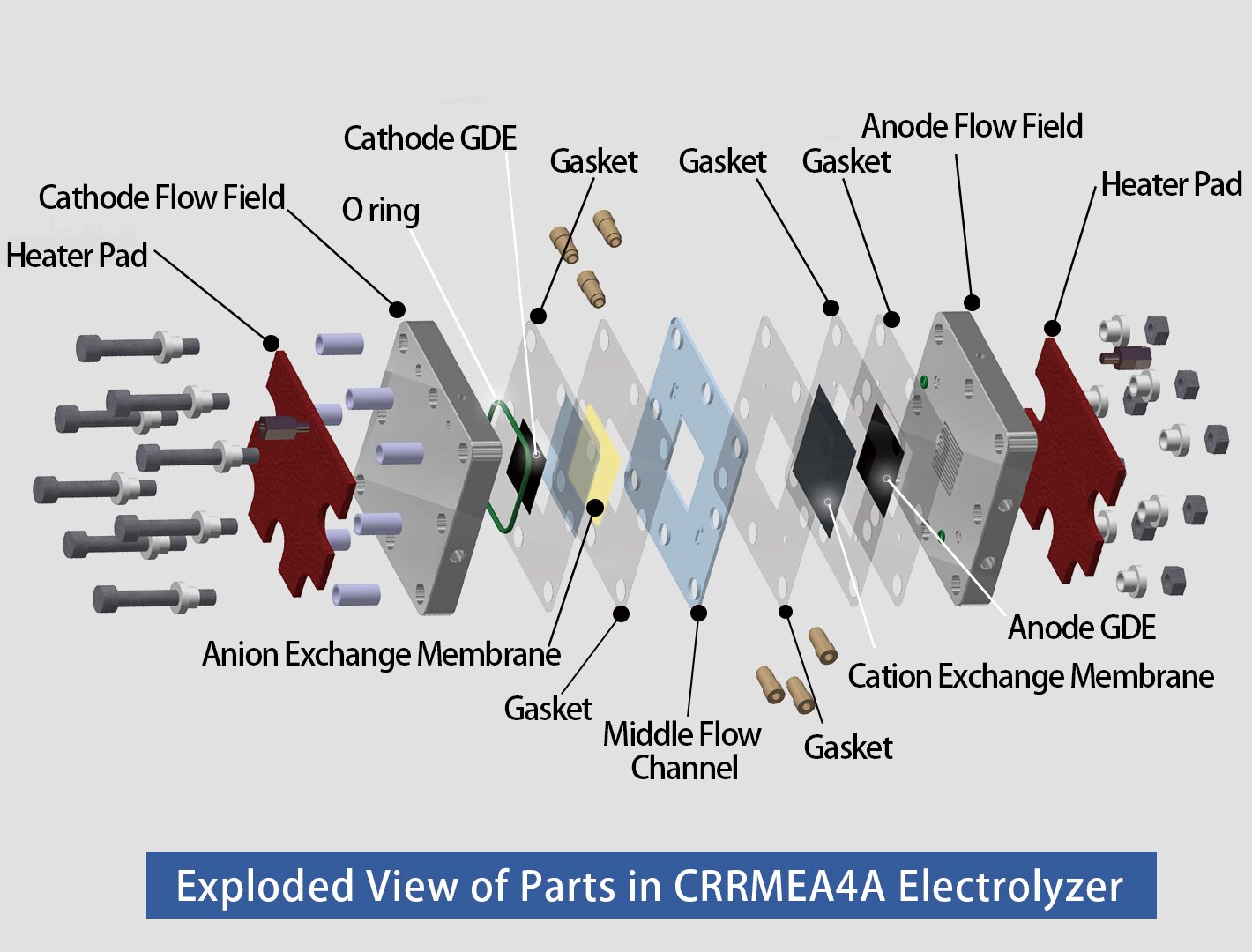
Fueiceel® Research Grade 4cm2 Electrolyzer to Convert CO2 to HCOOH is a precision-engineered device designed for the electrochemical conversion of CO2 into formic acid. This system utilizes a three-compartment design to optimize the conversion process. In the cathode compartment, humidified CO2 is electrochemically reduced to formate anions, while in the anode compartment, deionized (DI) water is electrolyzed to generate protons. The formate anions pass through an anion exchange membrane into the central compartment, while protons are transferred through a cation exchange membrane into the same compartment. The combination of formate anions with protons in this central compartment results in the formation of formic acid, which is collected at the outlet.
This 4 cm² electrolyzer includes a titanium anode flow field, a central compartment with ion exchange media, a stainless steel cathode flow field and all necessary accessories such as nuts, bolts, o-rings, gaskets, and an insulating kit.
A complete electrolyzer with ion exchange media (in central compartment), catalyst-coated electrodes, Nafion® and Sustainion® membranes are also available upon request. The complete electrolyzer is fully assembled, tested, and verified to ensure optimal performance before shipment.
 |  |
 |
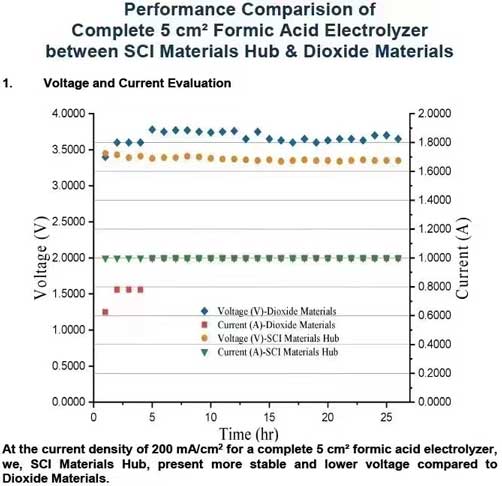
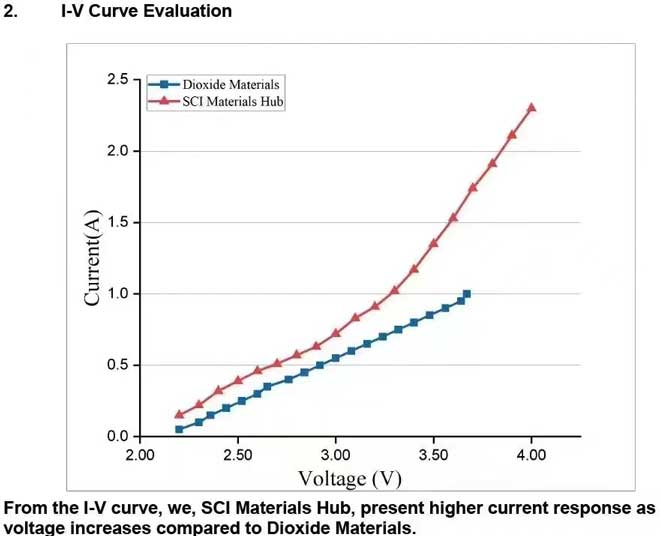
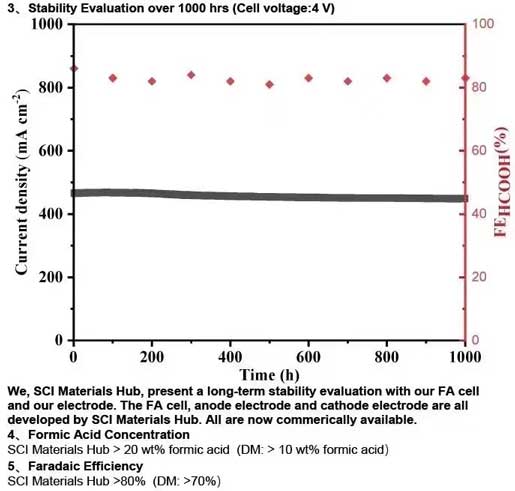
At the current density of 200 mA/cm² for a complete 5 cm² formic acid electrolyzer, we, SCI Materials Hub, present more stable and lower voltage compared to Dioxide Materials. We, SCI Materials Hub, present a long-term stability evaluation with our FA cell and our electrode. The FA cell, anode electrode and cathode electrode are all developed by SCI Materials Hub. All are now commerically available.
Formic Acid Concentration SCI Materials Hub > 20 wt% formic acid (DM: > 10 wt% formic acid)
To set up the system, begin by connecting a peristaltic pump to circulate DI water from a reservoir to the anode chamber at a flow rate of 3 mL/min. A second peristaltic pump should feed DI water to the central compartment at a suggested flow rate of 0.065 mL/min through the inlets located on the backside of the anode flow field. Adjusting the flow rate into the central compartment can vary the concentration of produced formic acid—typically, a slower flow rate favors the production of higher concentration formic acid, albeit with decreased Faradaic efficiency. It is recommended to use 1/8” OD, 1/16” ID PTFE tubing for all connections. Pure CO2 from a cylinder should be humidified using a bottle humidifier (sold separately) and then fed to the cathode chamber at a flow rate of 30 sccm.
Cathode: Remove the nuts from the compression fittings and extract the black rubber rods from the nuts. Insert the 1/8” OD PTFE tubing through the nuts, and then secure the nuts onto the compression fittings. Connect the tubing from the CO2 humidifier to the compression fitting at the top of the cathode (stainless steel flow field) and tighten the nuts by hand. Connect another piece of PTFE tubing to the bottom compression fitting on the cathode and route it to the catholyte collector, and then to the exhaust. Since CO is poisonous and H2 is flammable, ensure that the cathode gas product is not released into the lab or working area.
Anode: Remove the nuts from all compression fittings and the black rubber rods from the nuts. Insert the 1/8” OD PTFE tubing through the nuts, and secure the nuts onto the compression fittings. Follow the labels on the compression fittings to connect the tubing accordingly, and tighten the nuts by hand.
Locate the threaded hole for the wire connection on the top of the cell. Attach the ring terminal with a Phillips round head screw. Repeat this procedure for both the anode and cathode connections.
To begin testing, start the DI water flow from the reservoir to the inlet of the anode chamber at a rate of 3 mL/min, and to the central compartment at a flow rate of 0.065 mL/min. Feed humidified CO2 into the cathode chamber at a flow rate of 30 sccm. Connect the anode electrical lead (red) and cathode electrical lead (black) to the positive and negative terminals, respectively, on the power supply. Set the initial voltage to 4.5-5.0V and the current to 0.4A (0.1A/cm²). As the voltage stabilizes over time, gradually increase the current to 0.24A, 0.32A, and then 0.4A. The electrolyzer should reach stable operating conditions within several hours, depending on membrane and electrode conditioning.
For long-term testing, it is recommended to perform a reverse polarity treatment at 1.5V for 30 seconds approximately every 100 hours to maintain cell performance.
| Accessories | ||||
Cathode flow field |
FF411316a-S13 Stainless Steel |
FF411316a-TI2 Titanium | ||
Anode flow field |
FF411300a-TI2 Titanium | FF411300a-TI2P Platinized Titanium | ||
| Central compartment |
1mm |
1.5mm | ||
| Gasket |
PTFE gasket set for FA cell, USD$85/pc (100/200/250/300/400/500/1000μm available) |
FKM gasket set for FA cell, USD$85/pc (100/200/250/300/400/500/1000μm available) | ||
| Tube |
Teflon tube (ID1/16" OD1/8") (USD$10/m) |
Corrosion resistant tube for peristaltic pump (ID1.6mm/OD4.8mm) (USD$50/m) | ||
| Connectors |
PTFE bolts x6 (ID1/8"), $25/set |
PTFE bolts x6 (ID1/8"), $30/set |
Nickel bolts x6 (ID1/8"), $30/set | |
| Others |
Tighen-insulation kit, $10/pc |
SS springs, $2/set |
Torque wrench with sleeve (1-25 Nm), $100/set | |
25A High current DC electrical lead pair - Alligator Clip $10/pair/0.5m; $15/pair/1m $20/pair/1.5m; $25/pair/2m |
25A High current DC electrical lead pair - Banana plug to Alligator Clip $10/pair/0.5m; $15/pair/1m $20/pair/1.5m; $25/pair/2m |
35A High current DC electrical lead pair - Banana plug to Alligator Clip $15/pair/0.5m; 20/pair/1m $25/pair/1.5m; $30/pair/2m |
40A High current DC electrical lead pair - Ring to Ring $20/pair/0.5m; 25/pair/1m $30/pair/1.5m; $35/pair/2m | |
Temperature controller, $699 (Accuracy: 0.1°C) |
Heating pads ($97/pair) Heating pad binder, $50/25ml |
O rings, $2/set |
Wrench kit, $10/set | |
VHP01 vacuum heater |
Cu conductors, $1/set | Small peristaltic pump, $300/pc Standard peristaltic pump, $400/pc Standard peristaltic pump with two channels, $700/pc Gear pump, $700/pc | DC power supply with data recording, storage, and export functions, $1000/pc | |
Humidifier Kit, USD$24 |
Humidifier with 6 PSI safety valve, USD$69 | * Mass flow controller with reader (CO2, 500sccm), USD$1500 ** Mass flow controller with Modbus RS485 Communication (CO2, 500sccm), USD$2000 |
PP isodiametric barbed hose connector Hose IDΦ1-Φ1.6mm, USD$2/pc Hose IDΦ1.6-Φ2.4mm,USD$2/pc Hose IDΦ2.4-Φ3.2mm,USD$2/pc Hose IDΦ3.2-Φ4mm, USD$2/pc | |
PP barbed connector for variable diameter hoses Hose IDΦ1.6↔Φ2.4, USD$2/pc Hose IDΦ1.6↔Φ3.2,USD$2/pc Hose IDΦ2.4↔Φ3.2, USD$2/pc Hose IDΦ2.4↔Φ4,USD$2/pc Hose IDΦ3.2↔Φ4, USD$2/pc |
PE isodiametric quick connector Tube ODΦ3-Φ3mm, USD$2/pc Tube ODΦ3.2-Φ3.2mm,USD$2/pc Tube ODΦ4-Φ4mm, USD$2/pc Tube ODΦ6-Φ6mm,USD$2/pc |
PE quick connector for variable diameter tubes Tube ODΦ3-Φ3.2mm, USD$2/pc Tube ODΦ3-Φ4mm, USD$2/pc Tube ODΦ3-Φ5mm, USD$2/pc Tube ODΦ3-Φ6mm, USD$2/pc Tube ODΦ3.2-Φ4mm, USD$2/pc Tube ODΦ3.2-Φ6mm, USD$2/pc |  PTFE corrosion-resistant hose/tube adapter Tube ODΦ3.2mm↔hose IDΦ1.6mm, USD$10/pc Tube ODΦ3.2mm↔hose IDΦ2.4mm, USD$10/pc Tube ODΦ3.2mm↔hose IDΦ3.2mm, USD$10/pc Tube ODΦ3.2mm↔hose IDΦ4mm, USD$10/pc | |
PTFE corrosion-resistant isodiametric tube connector Φ3mm↔Φ3mm, USD$10/pc Φ3.2mm↔Φ3.2mm, USD$10/pc Φ4mm↔Φ4mm, USD$10/pc Φ6mm↔Φ6mm, USD$15/pc Φ8mm↔Φ8mm, USD$15/p |
PTFE corrosion-resistant connector for variable diameter tubes Φ3mm↔Φ3.2mm, USD$15/pc Φ3mm↔Φ4mm, USD$15/pc Φ3mm↔Φ6mm, USD$15/pc Φ3.2mm↔Φ4mm, USD$15/pc Φ4mm↔Φ6mm, USD$15/pc |
316L SS isodiametric tube connector Φ3mm↔Φ3mm, USD20/pc Φ3.2mm↔Φ3.2mm, USD20/pc Φ4mm↔Φ4mm, USD20/pc Φ6mm↔Φ6mm, USD20/pc Φ8mm↔Φ8mm, USD20/pc |  316L SS connector for variable diameter tubes Φ3mm↔Φ3.2mm, USD30/pc Φ3mm↔Φ4mm, USD30/pc Φ3mm↔Φ6mm, USD30/pc Φ4mm↔Φ6mm, USD30/pc | |
| Consumables | ||||
| AEM | PiperION A10R / PiperION A22R PiperION A13 / PiperION A17 PiperION A20 / PiperION A25 | NexIonic A20 | ||
| Ionomer | Fumion FAA-3 5wt% in ethanol | |||
| GDL | Youveim® Ni fiber paper | DM SS fiber paper Youveim® SS fiber paper | DiffuCarb® CP-A210R raw carbon paper DiffuCarb® CP-A330R raw carbon paper DiffuCarb® CP-A400R raw carbon paper DiffuCarb® CP-H450R raw carbon paper DiffuCarb® CP-H850R raw carbon paper | Youveim® Ti fiber paper Youveim® Ti screen Youveim® Platinized Ti fiber paper Youveim® Platinized Ti screen |
Anode Electrode | IrO2 | |||
| DM IrO2-carbon paper DiffuCarb® E300 IrO2-carbon paper DiffuCarb® E300T IrO2-carbon paper with hydrophobic interface DiffuCarb® E300H IrO2-carbon paper with hydrophilic interface | Youveim® E301T IrO2-SS fiber paper with hydrophobic interface Youveim® E301H IrO2-SS fiber paper with hydrophilic interface Youveim® E301PT IrO2-Platinized SS fiber paper Youveim® E301G IrO2-Gold Plated SS fiber paper | |||
Youveim® E303T IrO2-Ti fiber paper with hydrophobic interface Youveim® E303H IrO2-Ti fiber paper with hydrophilic interface Youveim® E303PT IrO2-Platinized Ti fiber paper Youveim® E303G IrO2-Gold Plated Ti fiber paper | Youveim® E305T IrO2-Nickel fiber paper with hydrophobic interface Youveim® E305H IrO2-Nickel fiber paper with hydrophilic interface Youveim® E305PT IrO2-Platinized Nickel fiber paper Youveim® E305G IrO2-Gold Plated Nickel fiber paper | |||
Youveim® E309 IrO2/Ti fiber paper Youveim® E310 IrO2/Platinized Ti fiber paper Youveim® E311 Pt-IrO2/Platinized Ti fiber paper | Youveim® E314 IrO2/Ti screen Youveim® E315 IrO2/Platinized Ti screen Youveim® E316 Pt-IrO2/Platinized Ti screen | |||
| Youveim® E320 IrO2/Ti foam Youveim® E321 IrO2/Platinized Ti foam Youveim® E322 Pt-IrO2/Platinized Ti foam | ||||
DiffuCarb® E330 Ir-carbon paper DiffuCarb® E330T Ir-carbon paper with hydrophobic interface DiffuCarb® E330H Ir-carbon paper with hydrophilic interface | Youveim® E340T Ir-Ti fiber paper with hydrophobic interface Youveim® E340H Ir-Ti fiber paper with hydrophilic interface Youveim® E341T Ir-Platinized Ti fiber paper with hydrophobic interface Youveim® E341H Ir-Platinized Ti fiber paper with hydrophilic interface | |||
Youveim® E343 Ir/Ti screen Youveim® E344 Ir/Platinized Ti screen Youveim® E345 Pt-Ir/Platinized Ti screen | Youveim® E347 Ir/Ti fiber paper Youveim® E348 Ir/Platinized Ti fiber paper Youveim® E349 Pt-Ir/Platinized Ti fiber paper | Youveim® E351 Ir/Ti foam Youveim® E352 Ir/Platinized Ti foam Youveim®E353 Pt-Ir/Platinized Ti foam | ||
Cathode Electrode | DM Ag-Carbon Paper DiffuCarb® E400 Ag-Carbon Paper | DM FA Cathode DiffuCarb® E410 Bi2O3 - carbon paper DiffuCarb® E411 Bi based composite - carbon paper | ||
For international orders, please ask us for quotes via
Email: contact@scimaterials.cn
Tel: +86 153-5789-9751
These cells are assembled by our engineers & a leakage-sealing test is done before leaving our lab. Customized electrochemical cells can be made upon requests.
The accessories include:
| Electrolyzer type | Components | Price (USD) |
| CRRMEA4A-1 | FF411316a-S13 Stainless steel cathode flow field plate (x1), FF411300a-TI2 Titanium anode flow field plate (x1) central compartment (x1), PTFE gasket set (x1), Stainless steel bolts+nuts+gaskets (x1 set), O-ring set (x1) Insulating sleeves+shoulder washers (x1 set)、PEEK bolts (x1 set) | $1798 |
| CRRMEA4A-2 | FF411316a-S13 Titanium cathode flow field plate (x1), FF411300a-TI2 Titanium anode flow field plate (x1) central compartment (x1), PTFE gasket set (x1), Stainless steel bolts+nuts+gaskets (x1 set), O-ring set (x1) Insulating sleeves+shoulder washers (x1 set)、PEEK bolts (x1 set) | $2398 |
| Complete FA electrolyzer | A complete electrolyzer with ion exchange media (in central compartment), catalyst-coated electrodes, Nafion® and Sustainion® membranes. The complete electrolyzer is fully assembled, tested, and verified to ensure optimal performance before shipment. | Add $3000 |
Partial references citing our materials (from Google Scholar)
Carbon Dioxide Reduction
1. ACS Nano Strain Relaxation in Metal Alloy Catalysts Steers the Product Selectivity of Electrocatalytic CO2 Reduction
The bipolar membrane (Fumasep FBM) in this paper was purchased from SCI Materials Hub, which was used in rechargeable Zn-CO2 battery tests. The authors reported a strain relaxation strategy to determine lattice strains in bimetal MNi alloys (M = Pd, Ag, and Au) and realized an outstanding CO2-to-CO Faradaic efficiency of 96.6% with outstanding activity and durability toward a Zn-CO2 battery.
2. Front. Chem. Boosting Electrochemical Carbon Dioxide Reduction on Atomically Dispersed Nickel Catalyst
In this paper, Vulcan XC-72R was purchased from SCI Materials Hub. Vulcan XC 72R carbon is the most common catalyst support used in the anode and cathode electrodes of Polymer Electrolyte Membrane Fuel Cells (PEMFC), Direct Methanol Fuel Cells (DMFC), Alkaline Fuel Cells (AFC), Microbial Fuel Cells (MFC), Phosphoric Acid Fuel Cells (PAFC), and many more!
3. Adv. Mater. Partially Nitrided Ni Nanoclusters Achieve Energy-Efficient Electrocatalytic CO2 Reduction to CO at Ultralow Overpotential
An AEM membrane (Sustainion X37-50 Grade RT, purchased from SCI Materials Hub) was activated in 1 M KOH for 24 h, washed with ultra-purity water prior to use.
4. Adv. Funct. Mater. Nanoconfined Molecular Catalysts in Integrated Gas Diffusion Electrodes for High-Current-Density CO2 Electroreduction
In this paper (Supporting Information), an anion exchanged membrane (Fumasep FAB-PK-130 obtained from SCI Materials Hub (www.scimaterials.cn)) was used to separate the catholyte and anolyte chambers.
SCI Materials Hub: we also recommend our Fumasep FAB-PK-75 for the use in a flow cell.
5. Appl. Catal. B Efficient utilization of nickel single atoms for CO2 electroreduction by constructing 3D interconnected nitrogen-doped carbon tube network
In this paper, the Nafion 117 membrane was obtained from SCI Materials Hub.
In this paper, Proton exchange membrane (Nafion 117), Nafion D520, and Toray 060 carbon paper were purchased from SCI Materials Hub.
7. National Science Review Confinement of ionomer for electrocatalytic CO2 reduction reaction via efficient mass transfer pathways
An anion exchange membrane (PiperION-A15-HCO3) was obtained from SCI Materials Hub.
8. Catalysis Communications Facilitating CO2 electroreduction to C2H4 through facile regulating {100} & {111} grain boundary of Cu2O
Carbon paper (TGPH060), membrane solution (Nafion D520), and ionic membrane (Nafion N117) were obtained from Wuhu Eryi Material Technology Co., Ltd (a company under SCI Materials Hub).
Batteries
1. J. Mater. Chem. A Blocking polysulfides with a Janus Fe3C/N-CNF@RGO electrode via physiochemical confinement and catalytic conversion for high-performance lithium–sulfur batteries
Graphene oxide (GO) in this paper was obtained from SCI Materials Hub. The authors introduced a Janus Fe3C/N-CNF@RGO electrode consisting of 1D Fe3C decorated N-doped carbon nanofibers (Fe3C/N-CNFs) side and 2D reduced graphene oxide (RGO) side as the free-standing carrier of Li2S6 catholyte to improve the overall electrochemical performance of Li-S batteries.
This paper used more than 10 kinds of materials from SCI Materials Hub and the authors gave detailed properity comparsion.
The commercial IEMs of Fumasep FAB-PK-130 and Nafion N117 were obtained from SCI Materials Hub.
Gas diffusion layers of GDL340 (CeTech) and SGL39BC (Sigracet) and Nafion dispersion (Nafion D520) were obtained from SCI Materials Hub.
Zn foil (100 mm thickness) and Zn powder were obtained from the SCI Materials Hub.
Commercial 20% Pt/C, 40% Pt/C and IrO2 catalysts were also obtained from SCI Materials Hub.
3. Journal of Energy Chemistry Vanadium oxide nanospheres encapsulated in N-doped carbon nanofibers with morphology and defect dual-engineering toward advanced aqueous zinc-ion batteries
In this paper, carbon cloth (W0S1011) was obtained from SCI Materials Hub. The flexible carbon cloth matrix guaranteed the stabilization of the electrode and improved the conductivity of the cathode.
4. Energy Storage Materials Defect-abundant commercializable 3D carbon papers for fabricating composite Li anode with high loading and long life
The 3D carbon paper (TGPH060 raw paper) were purchased from SCI Materials Hub.
5. Nanomaterials A Stable Rechargeable Aqueous Zn–Air Battery Enabled by Heterogeneous MoS2 Cathode Catalysts
Nafion D520 (5 wt%), and carbon paper (GDL340) were received from SCI-Materials-Hub.
Carbon cloth (W0S1011) and other electrochemical consumables required for air cathode were provided by SCI Materials Hub.
Oxygen Reduction Reaction
1. J. Chem. Eng. Superior Efficiency Hydrogen Peroxide Production in Acidic Media through Epoxy Group Adjacent to Co-O/C Active Centers on Carbon Black
In this paper, Vulcan XC 72 carbon black, ion membrane (Nafion N115, 127 μL), Nafion solution (D520, 5 wt%), and carbon paper (AvCarb GDS 2230 and Spectracarb 2050A-1050) were purchased from SCI Materials Hub.
2. Journal of Colloid and Interface Science Gaining insight into the impact of electronic property and interface electrostatic field on ORR kinetics in alloy engineering via theoretical prognostication and experimental validation
The 20 wt% Pt3M (M = Cr, Co, Cu, Pd, Sn, and Ir) were purchased from SCI Materials Hub. This work places emphasis on the kinetics of the ORR concerning Pt3M (M = Cr, Co, Cu, Pd, Sn, and Ir) catalysts, and integrates theoretical prognostication and experimental validation to illuminate the fundamental principles of alloy engineering.
Water Electrolysis
1. International Journal of Hydrogen Energy Gold as an efficient hydrogen isotope separation catalyst in proton exchange membrane water electrolysis
The cathodic catalysts of Pt/C (20 wt%, 2–3 nm) and Au/C (20 wt%, 4–5 nm) were purchased from SCI Materials Hub.
2. Small Science Silver Compositing Boosts Water Electrolysis Activity and Durability of RuO2 in a Proton-Exchange-Membrane Water Electrolyzer
Two fiber felts (0.35 mm thickness, SCI Materials Hub) were used as the porous transport layers at both the cathode and the anode.
3. Advanced Functional Materials Hierarchical Crystalline/Amorphous Heterostructure MoNi/NiMoOx for Electrochemical Hydrogen Evolution with Industry-Level Activity and Stability
Anion-exchange membrane (FAA-3-PK-130) was obtained from SCI Materials Hub website.
Fuel Cells
1. Polymer Sub-two-micron ultrathin proton exchange membrane with reinforced mechanical strength
Gas diffusion electrode (60% Pt/C, Carbon paper) was purchased from SCI Materials Hub.
Characterization
1. Chemical Engineering Journal Electrochemical reconstitution of Prussian blue analogue for coupling furfural electro-oxidation with photo-assisted hydrogen evolution reaction
An Au nanoparticle film was deposited on the total reflecting plane of a single reflection ATR crystal (SCI Materials Hub, Wuhu, China) via sputter coater.

|
We Provide A Broad Range of Materials, Instruments & Solutions in Advanced Science and Technologies | About Us |



