
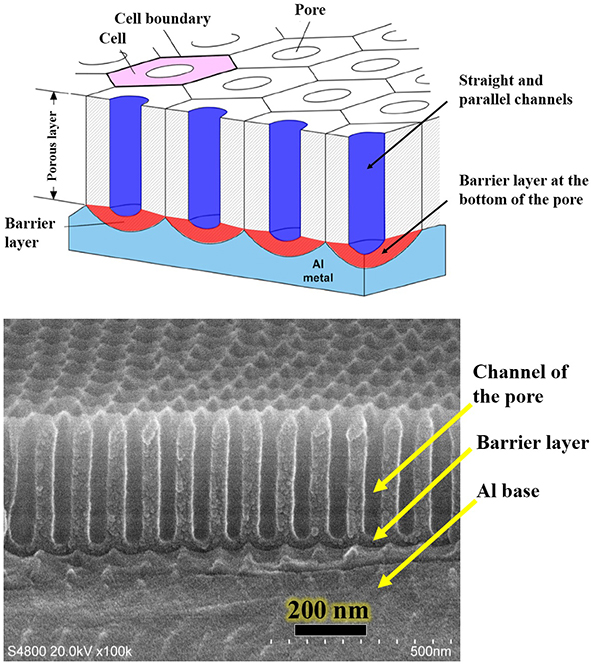
Figure 1. Schematic diagram of aluminum-based AAO template (porous nanotemplate) structure and SEM diagram
(Note: In order to simplify the model, in the upper schematic diagram of Figure 1, the front of the AAO is drawn as absolutely flat, in fact, there are bulges around each hole, and the microscopic upper surface is uneven)
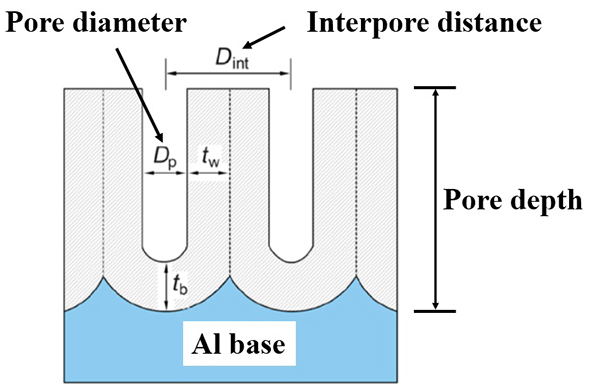
Figure 2.Cross-sectional diagram of an aluminum-based AAO template or porous nanotemplate
Aluminum-based AAO (Anodic Aluminum Oxide) template, also known as single-pass AAO template, it has a honeycomb structure (as shown in Figure 1), composed of many hexagonal cylindrical alumina (Al2O3) protocells, each protocell has a circular small hole in the middle, at the lower end of the hole there is a hemispherical barrier layer, the barrier layer below is aluminum-based, can also be called single-pass AAO or single-pass AAO template. Aluminum-based AAO is the simplest of the porous nanotemplate series, which is easy to prepare, has high yield, and can achieve uniform preparation of a large area of more than several hundred square centimeters. Figure 2 shows a cross-sectional diagram of an aluminum-based AAO template or a porous nanotemplate. According to the different preparation conditions, the pore size of single-pass AAO can achieve a pore size of tens of nanometers to hundreds of nanometers, and the pore depth (that is, the thickness of AAO on the AAO template) is continuously controllable, shallow tens of nanometers, and deep can reach tens of microns. The nanoscale pores on the aluminum-based AAO surface are arranged in a short range order, the order range is micron, and the arrangement is hexagonal dense structure. Its pore size is uniformly controllable over a wide area and can be used as a master to transfer porous structures to other materials. The high-density pore distribution and slender pores provide a three-dimensional nanostructure with a very high surface ratio, which can realize a large number of adsorption of organic molecules, and have good application prospects in such as optical detection.
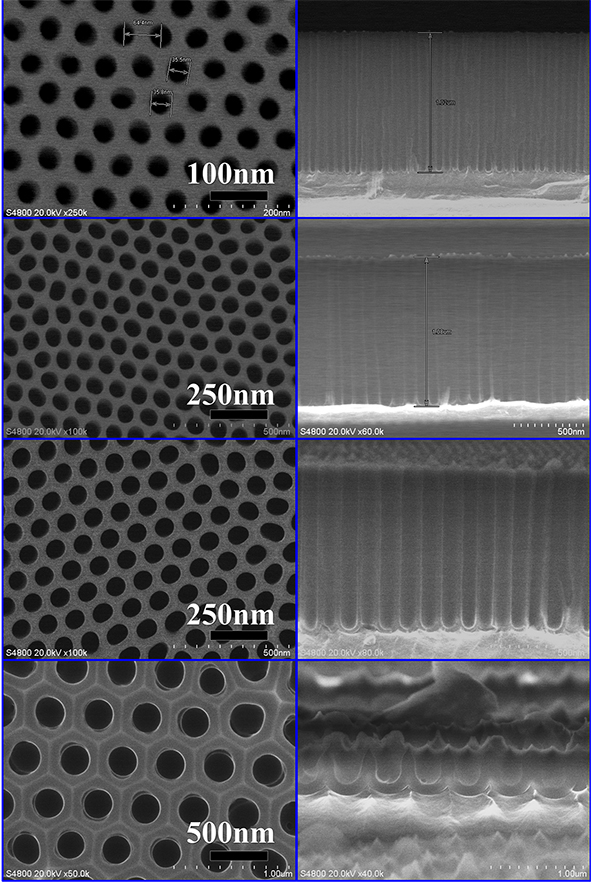
Figure 3. SEM diagram of aluminum-based AAO (porous nanotemplate).
The left side of Figure 3 shows the top view of several typical aluminum-based AAO SEM diagrams, and the right side is the cross-sectional view SEM diagram, it can be seen that the AAO template is hexagonally densely arranged in a small area, the aperture is relatively uniform, and the barrier layer at the bottom of the hole is clearly visible.

Figure 4.Actual diagram of aluminum-based AAO template (porous nano template) product
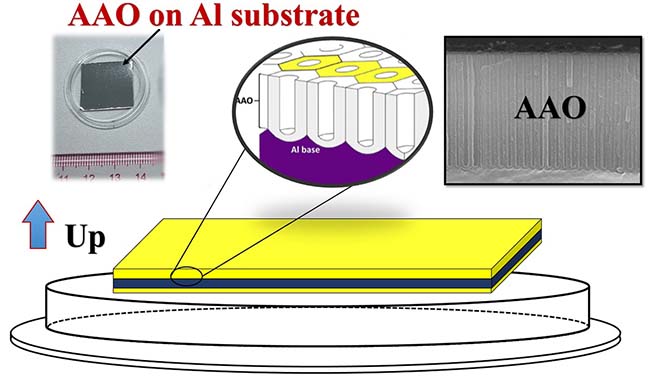
Figure 5. Schematic diagram of the placement direction of aluminum-based AAO products
The aluminum-based AAO template has a highly ordered porous structure on one side. Due to the support of the aluminum base, the aluminum base AAO has high strength, can be clamped with tweezers and slightly bent, and can be cleaned for a short time. Be careful not to touch the membrane surface directly with your fingers, otherwise grease or other foreign objects on your hands will easily get to the membrane surface and it will be very difficult to clean it.
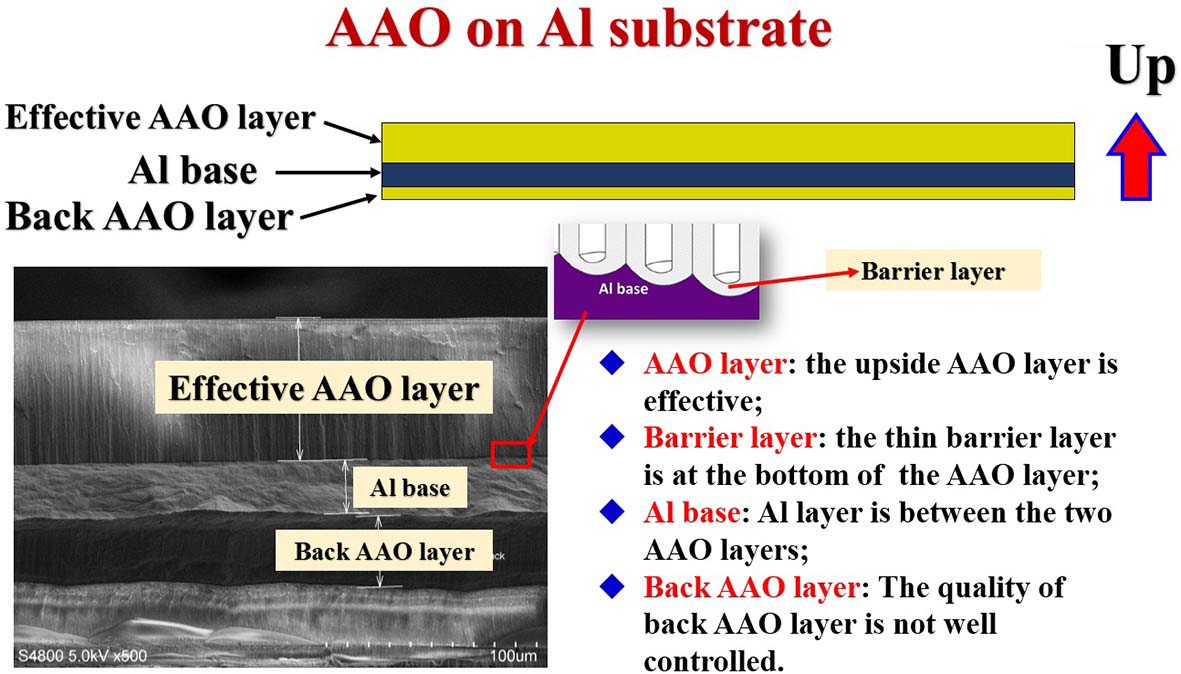
Figure 6. Schematic diagram of the structure of the aluminum-based AAO template
Aluminum-based AAO template product packaging box face up, the upper surface is an effective AAO layer, the back of the product also has a layer of porous alumina, but this layer of alumina is thinner than the effective AAO layer, the quality is poor, please do not use this layer of oxide as a porous template. Aluminum-based single-pass AAO template placement method is the front up towards the lid, if the appearance of the two sides is obviously different, the front side appears bright and uniform, the reverse side is rough and uneven, if the appearance of the two sides is exactly the same, it can not be distinguished by the naked eye, so in order to prevent confusion between the front and back, it is recommended to mark the back of the back with a marker when taking out the single-pass AAO. AAO membrane can be slowly removed with 5wt% phosphoric acid, or 5~10wt.% NaOH for rapid removal (under the premise of ensuring that the AAO part is easy to contact with phosphoric acid or NaOH).
About how to remove the aluminum base: If the aluminum-based AAO is used as a template to prepare polymer nanowires or nanorods, the solution of the polymer can be dropped on the AAO surface, or the polymer film can be spread on the AAO surface and then heated. After preparation, since the front side of AAO is protected by a polymer layer, the entire sample cannot be simply placed in NaOH to remove AAO. At this time, the oxide layer on the back side can be removed with NaOH, then the aluminum base is removed with copper chloride, and finally the AAO template layer can be quickly removed with NaOH.
Detailed operation instructions for aluminum base removal: The surface of our single-pass AAO and V-shaped AAO products is free of any polymer material. What is said here is that if you use a single-pass or V-type AAO process, if a layer of other materials is coated on its surface, such as polymer materials, due to the coverage of this layer of material, if the single-pass AAO is placed directly in the NaOH solution, this layer of material will isolate the AAO from the NaOH solution, so that the purpose of dissolving AAO cannot be achieved. Since the single-pass AAO is coated with polymer material on the front, the AAO template cannot be removed directly with NaOH solution, because AAO and NaOH are isolated by the polymer layer. So the AAO must be removed from the back.
(1) Remove the back oxide layer: the sample is face up, gently placed on the surface of the NaOH solution with a mass fraction of 5% (at room temperature), because the polymer layer is generally hydrophobic, so the sample will float on the surface of the solution (of course, sinking is okay, does not affect), NaOH will quickly (minutes to ten minutes) to dissolve the alumina layer on the back, so that the aluminum base is exposed, the exposed aluminum base will be in direct contact with NaOH, the production of hydrogen, you can see a lot of bubble production, Indicates that the alumina on the back is removed. Since the reaction is not too violent, the sample is generally not washed up. Remove the sample and rinse with water.
(2) Remove the aluminum base on the back: Since NaOH reacts very, very slowly with aluminum, NaOH is not used to remove aluminum. Preparation of copper chloride (CuCl2) hydrochloric acid solution, if there is no copper chloride can be used copper sulfate, solution concentration can be arbitrary, the principle is that the higher the concentration of copper chloride and hydrochloric acid, the faster the reaction, the more intense, the shorter the time, for example, you can use copper chloride with a mass fraction of 20%, and the volume concentration of hydrochloric acid can be 10%. The sample is placed on the surface of the solution, at this time, copper ions, hydrogen ions, aluminum will react, produce hydrogen, copper element, aluminum ions, when the concentration of copper chloride is high, the reaction is very violent, produce a large amount of heat, when the reaction is very violent, the bubbles will top the sample and shake violently. If you feel that the reaction is too violent, you can immediately pour ionized water into the solution to dilute the solution, and the reaction speed will drop immediately. If you feel that the reaction is too slow, you can add some copper chloride and hydrochloric acid to the solution. When the bubbles disappear, the aluminum reacts and the sample becomes transparent (if the polymer layer is colorless and transparent). At this time, the sample can be taken out and placed on the surface of a new cup of copper chloride hydrochloric acid solution for ten minutes, so that the residual invisible aluminum is completely removed. Remove the sample and wash it gently with water.
(3) The sample floats face-up on the surface of the NaOH solution of 5% of the mass molecule (at room temperature), under normal circumstances, the thickness of 5 microns below the single-pass AAO, ten or twenty minutes can completely disappear, if the thickness of tens of microns, can be appropriately extended to thirty minutes or even 1 hour, because NaOH and AAO reaction without bubble production, and no change, so the naked eye can not judge whether AAO is completely removed, and finally subject to SEM detection.
Prompt: When the front AAO is bare, how to remove the back AAO: AAO floats on the surface of NaOH solution, but it is necessary to first coat a small amount of hydrophobic material around the sample, such as nail polish, or directly stick to the edge of the front side with high-temperature tape, coating or pasting width 1~3mm, in order to prevent NaOH solution from spreading to the AAO on the front side when floating. When the AAO film is very thin (such as less than 2 microns), and it is necessary to remove the aluminum base, it is not recommended that the AAO surface be exposed, because the bubbles generated when removing the aluminum base are likely to break through the AAO film, so it is best to coat the front of the polymer protective film with a dozen to tens of microns. We do not recommend that you de-aluminum base single-pass AAOs with uncoated protective film on the surface.
Aluminum-based AAO templates can be characterized using SEM or AFM. Since AAO itself does not conduct electricity, when the thickness of AAO exceeds a few microns, please spray gold or carbon during the SEM test. When the AAO thickness is less than 1 micron, it can be directly observed without spraying gold or carbon. When observing the cross-section, the membrane can be gently bent to 90°, and a fresh section can be formed at the bend for observation. When the difference between the aperture and the hole spacing of the single-pass AAO template is small, it is difficult to form an observable section at the bend, and the bending method is not applicable. When the thickness of AAO reaches tens of microns, the bending method is also limited, because the crack formed after bending is relatively small, and the bottom of the AAO section may be partially blocked when observing, at this time, the bending angle should be larger, or it should be broken to observe the section. Single-pass AAO thickness has a certain error when measuring, due to the test angle and other reasons, and the film itself also has a certain thickness fluctuation, the test sometimes error can reach 10%, so the very accurate value of the film thickness needs to be detected by yourself.
Tips: AAO template is prepared by bottom-up method and belongs to self-organizing structure, so its pore size has a certain distribution range, rather than a single value, especially the template with a hole spacing of 450nm is more uniform. The holes are arranged in short-range orders (micron range), and each ordered region can be called a "chip", and the shape of the hole at the chip boundary may not be exactly circular. Ultrafilms have a wider pore size distribution than double-pass thick films and single-pass films. If you require a very high uniformity of pore size and a very high roundness of porous membranes, then AAO is not a good choice.
For AAO with a hole center spacing (such as a hole spacing of 450nm), the larger the hole, the shape of the hole is close to the circle, and the smaller the hole, the more the shape of the hole is deviated from the circle; The hole depth in the specification list is actually the thickness of the effective AAO, excluding the thickness of the back oxide layer and the middle aluminum layer. The total thickness is about 0.15~0.2mm.
About cutting: Single-pass AAO can be cut into smaller pieces and used, generally without scissors, which are easy to cause sample bending and membrane cracking. For single-pass AAO with a film thickness of no more than 5 microns, you can find a flat table top or a piece of glass, spread a dust-free cloth, and then buckle the single-pass AAO on the dust-free cloth, and use a ruler and utility knife to cut on the back without too much force. You can cut it off a few more times, or you can stop when you are about to cut off, and then break back and forth a few times to break.
Note: When the thickness of the single-pass AAO film is 40~60 microns, many cracks will appear on both sides of the cutting line during cutting, and the cracks will expand. Therefore, when cutting, you can not cut on the back, but cut from the front, that is, AAO front up with a dust-free cloth pad on the AAO surface, a ruler pressed on the dust-free cloth, along the ruler, with a fresh utility knife tip back and forth, must not press hard, but gently scratch back and forth, like sawing wood, after dozens of strokes the surface AAO will break, and then continue to scratch a few times, and then break back and forth a few times can be broken. Even if this is done, there will still be few cracks on both sides of the fracture, please be aware.
Single-pass AAO can be assured of ultrasonic cleaning, which can be cleaned with acetone, ethanol, isopropanol, and deionized water.
Concentrate: Before measuring the SEM of the section of single-pass AAO, it is recommended to spray gold or carbon to increase the conductivity, which is conducive to clear observation of the cross-section.
For internaltional orders, please ask us for quotes via
Email: contact@scimaterials.cn
Tel: +86 15375698751
Wechat: SCI-Materials-Hub
Clik here to put quick orders on our Alibaba shop
BSP Single-pass AAO Template (Film Thickness: 500nm) | |||
Product code | Aperture/hole center spacing | Price & Specifications | Inventory |
| 31101041 | 70nm/100nm | $34 (20*20mm) | Ask for quote |
| 31101057 | 50nm/125nm | $34 (20*20mm) | Ask for quote |
| 31101058 | 70nm/125nm | $34 (20*20mm) | Ask for quote |
| 31101059 | 100nm/125nm | $34 (20*20mm) | Ask for quote |
SCI Materials Hub Is Committed to Offering The Best Price & Customer Services! | |||
Worldwide shipping via DHL, SF-Express & other requested carriers.
Payments via Bank Transfer, Paypal, Credit card (via Alibaba), Alipay, Wechat-pay are accepted.
Partial references citing our materials (from Google Scholar)
Carbon Dioxide Reduction
1. ACS Nano Strain Relaxation in Metal Alloy Catalysts Steers the Product Selectivity of Electrocatalytic CO2 Reduction
The bipolar membrane (Fumasep FBM) in this paper was purchased from SCI Materials Hub, which was used in rechargeable Zn-CO2 battery tests. The authors reported a strain relaxation strategy to determine lattice strains in bimetal MNi alloys (M = Pd, Ag, and Au) and realized an outstanding CO2-to-CO Faradaic efficiency of 96.6% with outstanding activity and durability toward a Zn-CO2 battery.
2. Front. Chem. Boosting Electrochemical Carbon Dioxide Reduction on Atomically Dispersed Nickel Catalyst
In this paper, Vulcan XC-72R was purchased from SCI Materials Hub. Vulcan XC 72R carbon is the most common catalyst support used in the anode and cathode electrodes of Polymer Electrolyte Membrane Fuel Cells (PEMFC), Direct Methanol Fuel Cells (DMFC), Alkaline Fuel Cells (AFC), Microbial Fuel Cells (MFC), Phosphoric Acid Fuel Cells (PAFC), and many more!
3. Adv. Mater. Partially Nitrided Ni Nanoclusters Achieve Energy-Efficient Electrocatalytic CO2 Reduction to CO at Ultralow Overpotential
An AEM membrane (Sustainion X37-50 Grade RT, purchased from SCI Materials Hub) was activated in 1 M KOH for 24 h, washed with ultra-purity water prior to use.
4. Adv. Funct. Mater. Nanoconfined Molecular Catalysts in Integrated Gas Diffusion Electrodes for High-Current-Density CO2 Electroreduction
In this paper (Supporting Information), an anion exchanged membrane (Fumasep FAB-PK-130 obtained from SCI Materials Hub (www.scimaterials.cn)) was used to separate the catholyte and anolyte chambers.
SCI Materials Hub: we also recommend our Fumasep FAB-PK-75 for the use in a flow cell.
5. Appl. Catal. B Efficient utilization of nickel single atoms for CO2 electroreduction by constructing 3D interconnected nitrogen-doped carbon tube network
In this paper, the Nafion 117 membrane was obtained from SCI Materials Hub.
In this paper, Proton exchange membrane (Nafion 117), Nafion D520, and Toray 060 carbon paper were purchased from SCI Materials Hub.
7. National Science Review Confinement of ionomer for electrocatalytic CO2 reduction reaction via efficient mass transfer pathways
An anion exchange membrane (PiperION-A15-HCO3) was obtained from SCI Materials Hub.
8. Catalysis Communications Facilitating CO2 electroreduction to C2H4 through facile regulating {100} & {111} grain boundary of Cu2O
Carbon paper (TGPH060), membrane solution (Nafion D520), and ionic membrane (Nafion N117) were obtained from Wuhu Eryi Material Technology Co., Ltd (a company under SCI Materials Hub).
Batteries
1. J. Mater. Chem. A Blocking polysulfides with a Janus Fe3C/N-CNF@RGO electrode via physiochemical confinement and catalytic conversion for high-performance lithium–sulfur batteries
Graphene oxide (GO) in this paper was obtained from SCI Materials Hub. The authors introduced a Janus Fe3C/N-CNF@RGO electrode consisting of 1D Fe3C decorated N-doped carbon nanofibers (Fe3C/N-CNFs) side and 2D reduced graphene oxide (RGO) side as the free-standing carrier of Li2S6 catholyte to improve the overall electrochemical performance of Li-S batteries.
This paper used more than 10 kinds of materials from SCI Materials Hub and the authors gave detailed properity comparsion.
The commercial IEMs of Fumasep FAB-PK-130 and Nafion N117 were obtained from SCI Materials Hub.
Gas diffusion layers of GDL340 (CeTech) and SGL39BC (Sigracet) and Nafion dispersion (Nafion D520) were obtained from SCI Materials Hub.
Zn foil (100 mm thickness) and Zn powder were obtained from the SCI Materials Hub.
Commercial 20% Pt/C, 40% Pt/C and IrO2 catalysts were also obtained from SCI Materials Hub.
3. Journal of Energy Chemistry Vanadium oxide nanospheres encapsulated in N-doped carbon nanofibers with morphology and defect dual-engineering toward advanced aqueous zinc-ion batteries
In this paper, carbon cloth (W0S1011) was obtained from SCI Materials Hub. The flexible carbon cloth matrix guaranteed the stabilization of the electrode and improved the conductivity of the cathode.
4. Energy Storage Materials Defect-abundant commercializable 3D carbon papers for fabricating composite Li anode with high loading and long life
The 3D carbon paper (TGPH060 raw paper) were purchased from SCI Materials Hub.
5. Nanomaterials A Stable Rechargeable Aqueous Zn–Air Battery Enabled by Heterogeneous MoS2 Cathode Catalysts
Nafion D520 (5 wt%), and carbon paper (GDL340) were received from SCI-Materials-Hub.
Carbon cloth (W0S1011) and other electrochemical consumables required for air cathode were provided by SCI Materials Hub.
Oxygen Reduction Reaction
1. J. Chem. Eng. Superior Efficiency Hydrogen Peroxide Production in Acidic Media through Epoxy Group Adjacent to Co-O/C Active Centers on Carbon Black
In this paper, Vulcan XC 72 carbon black, ion membrane (Nafion N115, 127 μL), Nafion solution (D520, 5 wt%), and carbon paper (AvCarb GDS 2230 and Spectracarb 2050A-1050) were purchased from SCI Materials Hub.
2. Journal of Colloid and Interface Science Gaining insight into the impact of electronic property and interface electrostatic field on ORR kinetics in alloy engineering via theoretical prognostication and experimental validation
The 20 wt% Pt3M (M = Cr, Co, Cu, Pd, Sn, and Ir) were purchased from SCI Materials Hub. This work places emphasis on the kinetics of the ORR concerning Pt3M (M = Cr, Co, Cu, Pd, Sn, and Ir) catalysts, and integrates theoretical prognostication and experimental validation to illuminate the fundamental principles of alloy engineering.
Water Electrolysis
1. International Journal of Hydrogen Energy Gold as an efficient hydrogen isotope separation catalyst in proton exchange membrane water electrolysis
The cathodic catalysts of Pt/C (20 wt%, 2–3 nm) and Au/C (20 wt%, 4–5 nm) were purchased from SCI Materials Hub.
2. Small Science Silver Compositing Boosts Water Electrolysis Activity and Durability of RuO2 in a Proton-Exchange-Membrane Water Electrolyzer
Two fiber felts (0.35 mm thickness, SCI Materials Hub) were used as the porous transport layers at both the cathode and the anode.
3. Advanced Functional Materials Hierarchical Crystalline/Amorphous Heterostructure MoNi/NiMoOx for Electrochemical Hydrogen Evolution with Industry-Level Activity and Stability
Anion-exchange membrane (FAA-3-PK-130) was obtained from SCI Materials Hub website.
Fuel Cells
1. Polymer Sub-two-micron ultrathin proton exchange membrane with reinforced mechanical strength
Gas diffusion electrode (60% Pt/C, Carbon paper) was purchased from SCI Materials Hub.
Characterization
1. Chemical Engineering Journal Electrochemical reconstitution of Prussian blue analogue for coupling furfural electro-oxidation with photo-assisted hydrogen evolution reaction
An Au nanoparticle film was deposited on the total reflecting plane of a single reflection ATR crystal (SCI Materials Hub, Wuhu, China) via sputter coater.

|
We Provide A Broad Range of Materials, Instruments & Solutions in Advanced Science and Technologies | About Us |



